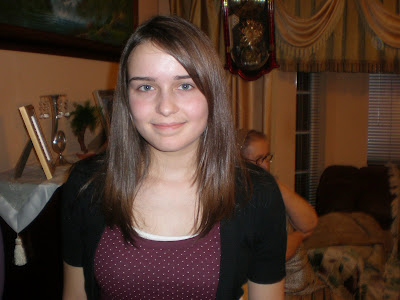This week is National Chemistry Week in honor of Mole Day tomorrow (10-23). One mole of a substance is 6.02 x 10^23 particles of that substance. So one mole of water would be 6.02 x 10^23 molecules of water which, as it turns out, is 18.0g of water. This can be figured out using the periodic table of elements using the atomic mass of any of the elements. The atomic mass tells us that one mole of that element is that many grams. So for oxygen it's 16.0g and for hydrogen it's 1.0g, but there are 2 hydrogens in water, so we multiply 1.0g x 2 and add 16.0g to get 18.0g in one mole of water. AMAZING!
In honor of National chemistry week and Mole Day, the PLU Chemistry Club put on "Mole Day Eve" for the PLU students and anyone who wanted to come. There was liquid nitrogen ice cream, other desserts (but who can top liquid nitrogen ice cream?) and of course . . . DEMOS! There were some stations set up for hands-on demos, but the professors also did cool (dangerous!) demos. I forgot my camera, but I did take pictures on my phone. They're not as good quality as from a camera, but you'll get the main point.

This is taken from the top of the stairs. People enjoying desserts!

That's how liquid nitrogen ice cream is made!

Liquid nitrogen everyone! It has to be stored in a special container because it's so cold. When you pour it out, it evaporates because it's boiling point is below room temperature, so the fog you are seeing is actually water vapor condensing because of the coldness of the liquid nitrogen.

Liquid nitrogen! Really you don't eat the nitrogen, you only use it to get the ice cream really cold. And the "ice cream" is milk, sugar, half-and-half and sometimes oreo cookies.

The finished product!

They put dry ice into the punch to keep it cold. The fog creates a nice effect!

Fluorescent stuff! The yellow on the sides is made with dish soap and flourenol, the blue is made with quinine and the center tube is a mix of the two. There's a fluorescent lamp that's shining on them to make them glow.

Fog! Creepy! We filled a lot of the sinks with hot water and added dry ice and viola!

Glow-in-the-dark Gak! It's made using glue, water and somethings else, plus fluorenol to make it glow in the dark. Kind of home made putty.

Ooblek! Made using starch and water! It's really cool and fun to play in! It's a liquid and a solid at the same time! Crazy! If you hit it or use force, it acts like a solid, but if you slowly work with it, it acts as a liquid. You can slowly sink your fingers in it, but if you try to pull them out really quickly, it's really hard! This happens because the starch molecules are kind of like fibers and the water molecules can ease them around something when you work slowly with it, but if you hit it, the starch molecules push out the water and become like a solid.

Elephant Toothpaste . . . coming out of a pumpkin! It's pretty cool. I think there's dish soap, hydrogen peroxide (30%) and IKI is the catalyst that starts the whole thing going. hydrogen peroxide decomposes to hydrogen and oxygen gas and makes the dish soap bubble.

The Screaming Gummy Bear! Some sort of inorganis solid was melted with a blow torch and a gummy bear was dropped into the melted substance. There was this sound (presumably the gummy bear screaming) and the purplish light and a lot of smoke! Hopefully we didn't set of the fire alarm! (We didn't)

Tinkerbell! Magnesium metal was put on a sheet of dry ice and lit with propane. It was then covered with another sheet of dry ice and the result was this really powerful light and it just went at it! It was really cool!
There were a few other demos, but it was hard to take a picture, because they were instantaneous. It was a really fun night!
 Jacob! He's so adorable! He loves watching Barbie and the Diamond Castle so we turned it on and got the kids to sit still for a little bit. Some adults watched it with the kids as well. I personally love the movie and I bought the DVD and now Jacob watches it all the time and keeps bugging me to buy him the princess' from the movie. He's so cute!
Jacob! He's so adorable! He loves watching Barbie and the Diamond Castle so we turned it on and got the kids to sit still for a little bit. Some adults watched it with the kids as well. I personally love the movie and I bought the DVD and now Jacob watches it all the time and keeps bugging me to buy him the princess' from the movie. He's so cute!
 Me, mom, maternal grandma, paternal grandma. . . both grandmas live with us and both of my grandpas died
Me, mom, maternal grandma, paternal grandma. . . both grandmas live with us and both of my grandpas died The guys were in my room most of the night playing computer games!
The guys were in my room most of the night playing computer games!